L-Dex® Score
ImpediMed devices incorporate L-Dex technology and bioimpedance spectroscopy (BIS) to measure and monitor fluid status—non-invasively, easily, quickly and accurately. The L-Dex score represents the difference in the amount of extracellular fluid in an at-risk limb compared to an unaffected limb.
How it works
Using BIS technology, the ImpediMed device sends a low-level electrical signal through the body. As lymphedema develops, the amount of fluid will increase, making it easier for the signal to travel though the extracellular fluid of the body.
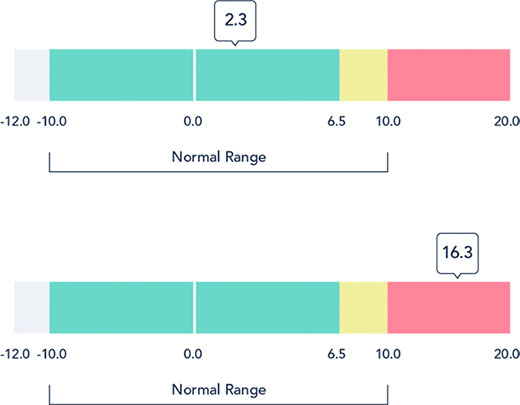
The L-Dex score compares how easily the electrical signal moves through the unaffected and the affected limbs. The L-Dex score is initially displayed against a normal or healthy range, as shown. For patients with a baseline measurement, subsequent L-Dex scores can be compared to their baseline for an even more personalized assessment. At-risk patients receive a baseline measurement before treatment and then are measured regularly after treatment. An L-Dex increase of 6.5 or more is an indication that lymphedema is developing, and intervention is needed.
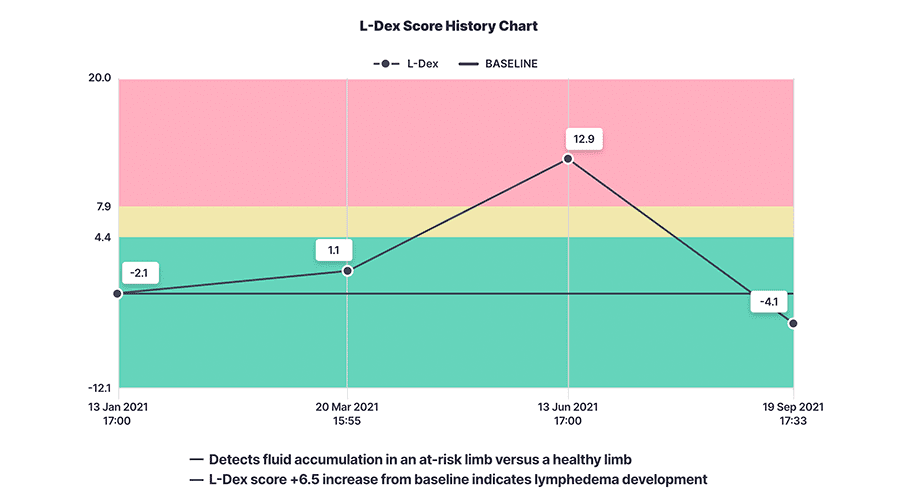
L-Dex in clinical practice
Lymphedema is rarely diagnosed until the patient experiences symptoms like pain, tightness, or swelling in their limb. However, with early diagnosis and intervention, the condition can be well managed1-3. By identifying the patient’s baseline L-Dex score and then measuring it at regular intervals post-surgery, healthcare professionals can accurately monitor patient progress and offer appropriate education and intervention to prevent the progression of lymphedema. Recently published results from the PREVENT Trial showed that 92% of patients with early detection using L-Dex and intervention did not progress to chronic lymphedema through three years.4
Clinical Evidence
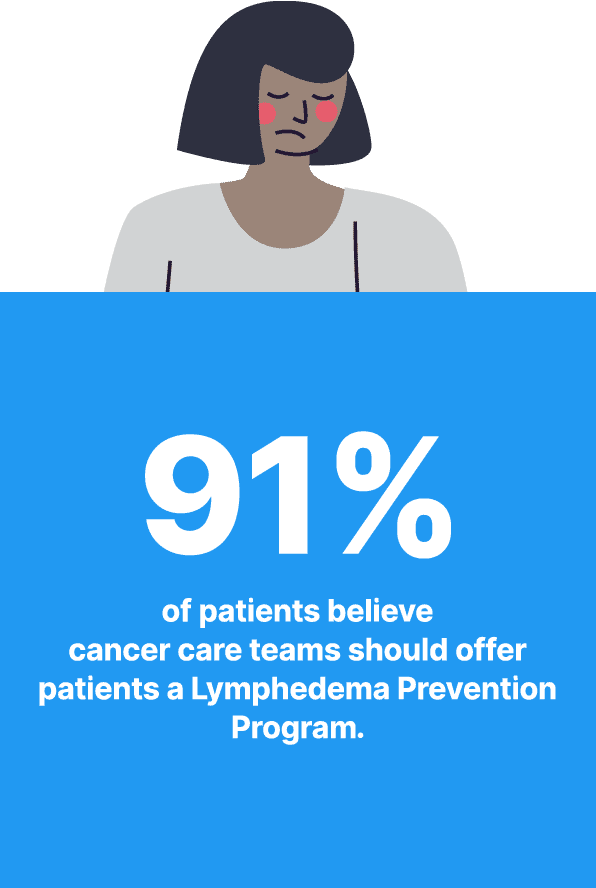
Why Expanding Access to a Lymphedema Prevention Program is Needed for Cancer Patients
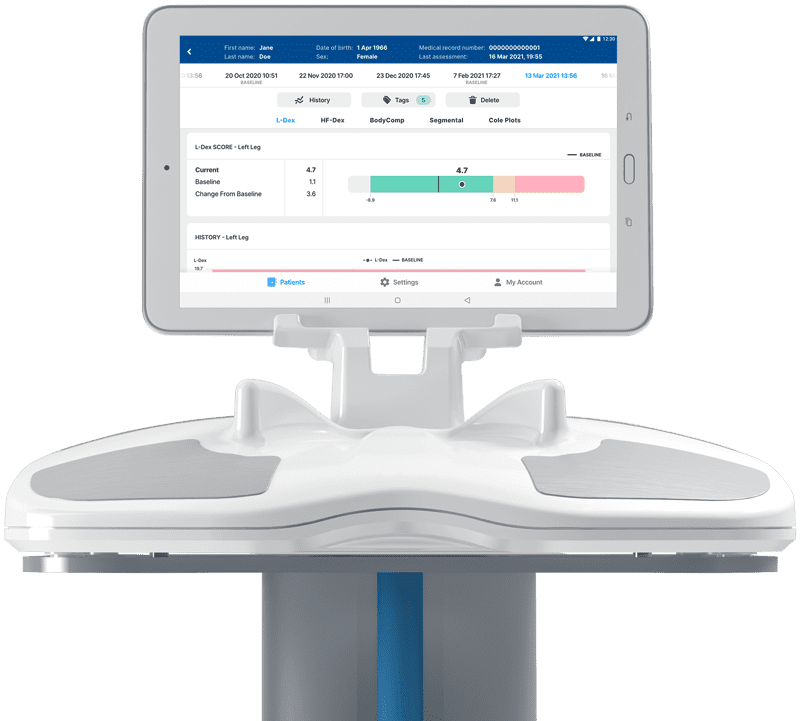
SOZO Digital Health Platform
SOZO uses ImpediMed’s BIS technology to measure and track critical information about the human body to aid clinicians in managing chronic disease and maximizing patient health. SOZO is non-invasive, fast, and easy to use. Results from the 30-second test* are available immediately providing proactive patient care including early detection of disease progression, treatment monitoring, and patient education.
*As tested on Android
Contact UsReferences
- Hayes S, Cornish B, Newman B. Comparison of methods to diagnose lymphedema among breast cancer survivors: 6-month follow up. Breast Cancer Res Treat. February 1989;(3):221-226.
- Czerniec S, et al. Assessment of lymphedema using measurement tools and self-report. 7th National Lymphedema Network Conference; 2006; Nashville, TN, USA.
- Cornish BH, et al. Early diagnosis of lymphedema using multiple frequency bioimpedance. Lymphology. 2001;34:2-11.
- Ridner SH, et al. A Comparison of Bioimpedance Spectroscopy or Tape Measure Triggered Compression Intervention in Chronic Breast Cancer Lymphedema Prevention. Lymphatic Research and Biology 2022.