ImpediMed Announces Next Generation of SOZO® Digital Health Platform Software
Major SOZO Update Helps Clinicians Measure Body Composition, Monitor Fluid Status and Assess Lymphedema in Patients
CARLSBAD, Calif., June 7, 2021 /PRNewswire/ — ImpediMed Limited (ASX:IPD), a global medical technology company using bioimpedance spectroscopy (BIS) to generate powerful data to improve patient health, today announced the release of the next generation software for the SOZO® Digital Health Platform.
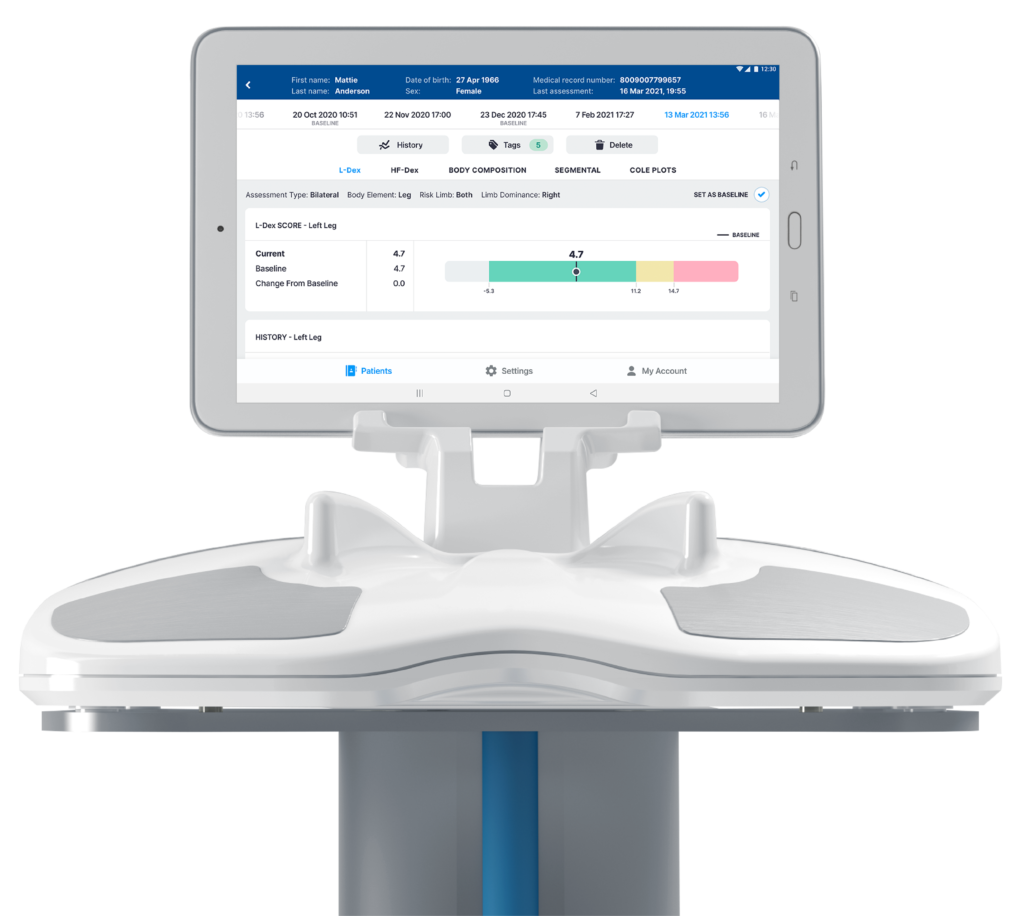
This software release, designated as Version 4.0, adds an intuitive redesign, enhanced security and privacy measures, and personalized data sets to provide clinicians with in-depth, actionable information to maximize patient health.
“The latest update to the SOZO Digital Health Platform provides clinicians with even more power to monitor patient health and make crucial clinical care decisions.
Version 4.0 allows care teams to generate personalized patient datasets, compare previous measurements with current ones, and add notes and tags to patient profiles,” said Richard Carreon,
Managing Director and CEO of ImpediMed. “At ImpediMed, our mission is to not only develop technologies to help clinicians in monitoring and determining the best course of treatment for their patients, but to advance and adapt our technologies to better suit the needs of the clinicians and their patients.”
The SOZO Digital Health Platform has gained broad adoption worldwide. Over 700 devices are in use by large and small healthcare systems as well as by AstraZeneca in support of clinical trials. The software improvements in this release are supported by clinical experience from over 250,000 SOZO patient tests and extensive human factors testing. SOZO’s cloud-based software solution provides customers with immediate access to updates, which take only minutes to complete.
The SOZO Digital Health Platform Version 4.0 software includes advancements such as a major redesign of the User Interface, making the software even more intuitive and easier to use for clinicians managing multiple patients across a variety of conditions. The visual display incorporates further customization, enabling clinicians to tag measurements and make patient notes supporting clinical decision making while assisting clinicians in the timely retrieval of critical information.
The upgraded software also features new tools to monitor patients’ body compositions. Novel reference ranges have been added to the body composition analysis, combining tissue and fluid analyses into one. An update to the segmental analysis implements the SOZO Digital Health Platform’s new license for measuring composition of the arms and legs. Version 4.0 also includes enhanced privacy and security features such as a hidden patient list, Multi Factor Authentication (MFA), and Single Sign On (SSO) to protect patient data.
The SOZO® device is a noninvasive monitoring tool using bioimpedance spectroscopy (BIS) technology to deliver a precise snapshot of fluid status and tissue composition in less than 30 seconds with 256 unique data points. The FDA-cleared, CE-marked and ARTG-listed digital health platform assists clinicians in the early detection of secondary lymphedema and measures fluid status for patients living with heart failure. It provides a patient’s L-Dex® score, the only non-invasive, reliable, validated tool to help clinicians identify subclinical lymphedema.
About ImpediMed
Founded and headquartered in Brisbane, Australia with U.S. offices in Carlsbad, Calif., and Bloomington, Minn., ImpediMed is the world leader in the development and distribution of medical devices employing bioimpedance spectroscopy (BIS) technologies for use in the noninvasive clinical assessment and monitoring of fluid status in patients. ImpediMed has the first medical device with FDA clearance in the U.S. to aid healthcare professionals to clinically assess secondary unilateral lymphedema of the arm and leg in women and the leg in men. For additional information, visit www.impedimed.com.